The approach to patients with established coronary artery disease (CAD)has been make progress including lifestyle, supplemental, and Rx approaches but more “tools” in the tool box are still needed. The explosion in overweight and obese heart patients is a major challenge.
Semaglutide (knows as Wegovy for weight loss), a glucagon-like peptide-1 receptor agonist, has been shown to reduce the risk of adverse cardiovascular events in patients with diabetes. Whether semaglutide (Wegovy) can reduce cardiovascular risk associated with overweight and obesity in the absence of diabetes is unknown and a new study reported their findings in a huge study of high quality.
STUDY METHODS
In a multicenter, double-blind, randomized, placebo-controlled, event-driven superiority trial, the researchers enrolled patients 45 years of age or older who had preexisting cardiovascular disease and a body-mass index (BMI) of 27 or greater but no history of diabetes.
Cardiovascular disease was defined as previous myocardial infarction, previous stroke, or symptomatic peripheral arterial disease.
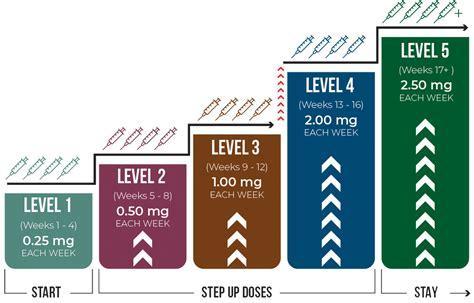
Patients were randomly assigned in a 1:1 ratio to receive once-weekly subcutaneous semaglutide at a dose of 2.4 mg or placebo. The starting dose of semaglutide was 0.24 mg once weekly, and the dose was increased every 4 weeks (to once-weekly doses of 0.5, 1.0, 1.7, and 2.4 mg) until the target dose of 2.4 mg was reached after 16 weeks.
The primary cardiovascular end point was a composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke in a time-to-first-event analysis. Safety was also assessed.
STUDY RESULTS
A total of 17,604 patients were enrolled; 8803 were assigned to receive semaglutide and 8801 to receive placebo. The mean duration of exposure to semaglutide or placebo was 34±14 months, and the mean duration of follow-up was 40 months. A primary cardiovascular end-point event occurred in 569 of the 8803 patients (6.5%) in the semaglutide group and in 701 of the 8801 patients (8.0%) in the placebo group (a 20% relative reductions in the semiglutide group).
Adverse events leading to permanent discontinuation of the trial product occurred in 1461 patients (17%) in the semaglutide group and 718 patients (8%) in the placebo group (P<0.001). Serious adverse events were reported in 2941 patients (33%) in the semaglutide group and 3204 patients (36%) in the placebo group (P<0.001).
In the group that discontinued the Rx, events included gastrointestinal disorders in 880 patients (10%) in the semaglutide group and 172 patients (2%) in the placebo group (P<0.001) and gallbladder-related disorders in 246 patients (3%) and 203 patients (2%), respectively (P=0.04).
STUDY CONCLUSIONS
In patients with preexisting cardiovascular disease and overweight or obesity but without diabetes, weekly subcutaneous semaglutide titrated up to a dose of 2.4 mg was superior to placebo in reducing the incidence of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke at a mean follow-up of 40 months. There were more side effects leading to discontinuation in the group treated with semiglutide (Wegovy).
These results are important. The use of semiglutide is already widespread but will not become a tool used by cardiologists and other practitioners. Side effects will need to monitored. Attempts at lifestyle like whole food plant diets, fasting mimicking diets, and exercise remain high priority.

